Diagnosed with advanced metastatic prostate cancer?
Overview by
Martin J Connor
Senior Clinical Research Fellow
& Urology Specialist Registrar
Imperial College NHS Trust

Professor Hashim Ahmed
Chair in Urology and Consultant Urological Surgeon
Imperial College NHS Trust

The information on this page is relevant if you have had a recently been diagnosed with metastatic prostate cancer, that is prostate cancer that has spread well beyond the prostate gland itself, into other areas of the body. This is called advanced or in medical terms “metastatic” prostate cancer (Figure 1). In particular, this page is highly relevant to men who have not had any prior prostate cancer diagnosis or treatment (often known as “de novo” or “synchronous” metastatic prostate cancer). This is staged as “M1” disease on the TNM staging
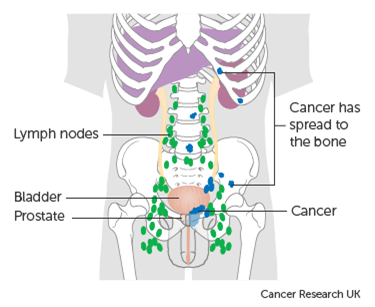
Figure 1. Advanced (metastatic) prostate cancer staging.
Legend: Metastatic prostate cancer is staged as M1a (cancer in distant non-pelvic lymph nodes); M1b (cancer in bone); and / or M1c (cancer in organs e.g. Lung, Liver).
Treatment pathway overview
The treatment options provided here are designed to provide you with a guide, ultimately the correct treatment decision will be made in discussion with your treating clinician and other members of the prostate cancer multi-disciplinary team (MDT). However, an overview of the possible treatment paths is provided in Figure 2.
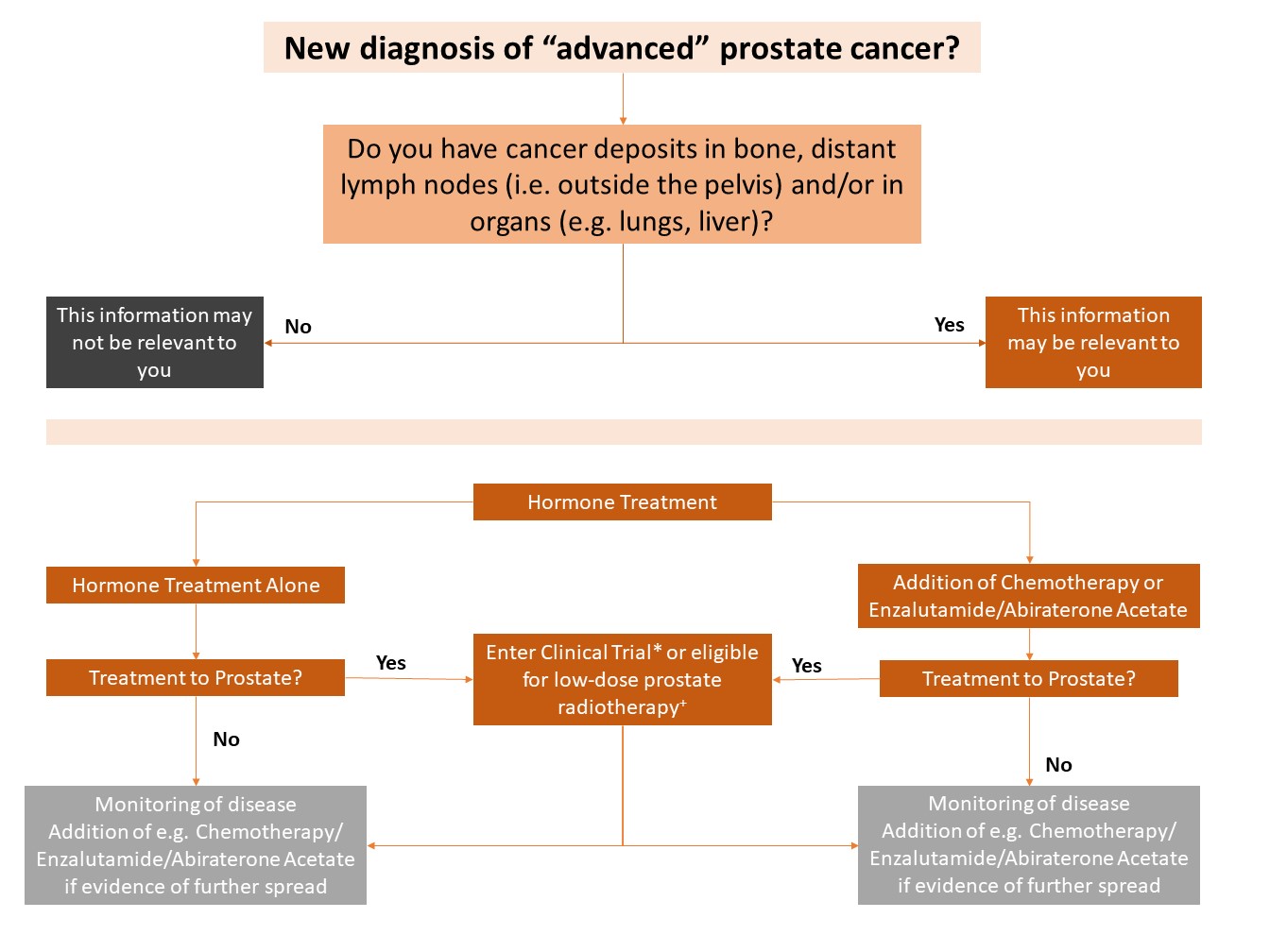
Figure 2. Treatment pathway in men with a new diagnosis of metastatic prostate cancer.
Legend: *NHS clinical trials, such as IP2-ATLANTA, are offering a range of treatment options (radiotherapy, surgery or ablation) to the prostate. +Low-dose prostate radiotherapy may be offered to you if your have a few or limited number of cancer deposits (oligometastatic).
(A) Medical treatment
Medical therapy involves hormone therapy and some men will be offered chemotherapy or a new hormone drug therapy (such as Enzalutamide) immediately following their diagnosis.
- i) Hormone Therapy (Androgen Deprivation Therapy)
Hormone therapy works by preventing your body producing testosterone and by stopping testosterone reaching cancer cells. Prostate cancer uses testosterone to grow. Hormone therapy once commenced in metastatic prostate cancer is normally lifelong. This will help control the spread of prostate cancer and prevent symptoms (such as bone pain) but does not provide a cure. There are many different formulations of hormone therapy, commonly your clinician will commence a long-acting treatment (such as luteinizing hormone-releasing hormone [LHRH] agonists). These hormones can be delivered by simple injection or implant once every three months.
Hormone therapy side effect vary, but may include weight gain, hot flushes, tiredness, loss of sexual desire (libido), difficulty in maintaining erections, muscle loss, breast tenderness, bone thinning and mood changes. You should discuss these with your clinician or cancer nurse specialist.
- ii) Chemotherapy (Docetaxel)
In addition to hormone therapy, you may be offered chemotherapy shortly after diagnosis. The drug “Docetaxel” is the chemotherapy that is approved for newly diagnosed metastatic prostate cancer. Chemotherapy results in death of cancer cells (cytotoxic). This will aim to control the spread of cancer and also treat existing deposits.
Recent large clinical trials have reported that, compared to hormone therapy alone, men treated with Docetaxel chemotherapy combined with hormone therapy demonstrated benefits in terms of added years of life and control of the disease. However, similar to hormone therapy this is not curative treatment.
NHS England reviewed the evidence in 2016 and concluded that was sufficient evidence to permit Docetaxel chemotherapy to be routinely funded for the treatment of newly diagnosed metastatic prostate cancer, where treatment with Docetaxel chemotherapy is started within 12 weeks of commencing hormone therapy.
Docetaxel (Taxotere®) chemotherapy is delivered under the care of a medical oncologist and usually takes the form of six cycles (or injections) of chemotherapy, given every three weeks. You will be asked to take oral steroid and/or an injection to maintain your white blood cell count (Granulocyte Colony Stimulating Factor [GCSF]) with each cycle. A blood test is routinely performed prior to each cycle starting.
Unfortunately, chemotherapy can also damage healthy cells and thus results in side-effects and these may include a high risk of infection, tiredness, nausea and vomiting, ankle swelling, numbness and tingling in your hands and feet. You should discuss these with your clinician or cancer nurse specialist.
iii) New drug therapy (Abiraterone Acetate, Enzalutamide)
A number of new tablet drugs have been developed as an alternative to chemotherapy. These new drugs act on hormone receptors. These medications have been previously approved in later stages of advanced prostate cancer, when hormone therapy is no longer effective (castrate-resistant disease). However, a number of large clinical trials have now demonstrated they such tablet therapy may offer similar benefits as Docetaxel chemotherapy, when used as a direct alternative.
Due to the expense of these drugs they have not been routinely available on the NHS. However, some of these medications are temporarily available, due to the covid-19 pandemic, depending on where you live in the UK. These rules are subject to changes frequently and should be discussed with your medical oncologist/clinician.
England & Wales NHS:
- Enzalutamide (Xtandi®) temporarily approved during COVID-19 only.
Scotland NHS:
- Abiraterone Acetate (Zytiga®) routinely approved.
(C) Clinical trials and new treatments options
- i) Treating the prostate despite spread of cancer
Despite the medical treatments available, the main cancer in the prostate is not normally treated as it was unclear the benefit from doing so. However, there is new evidence that some men who had a few or limited number of deposits (oligometastatic prostate cancer) may benefit from treating the main cancer inside the prostate in addition to the previously mentioned medical treatments. This is based on the theory that we might reduce the amount of cancer cells in the rest of the body and remove the cells that can grow despite. Further there is evidence that main cancer in the prostate can send growth signals to the areas of cancer outside the prostate.
There are a number of different trials open in the UK (e.g. IP2-ATLANTA) offering treatment of the prostate and/or lymph nodes. These trials offer traditional treatments such as radiotherapy and surgery (prostatectomy). In addition, some offer minimally-invasive freezing (cryotherapy) or heating (HIFU) to the prostate which may have fewer side-effects.
These treatments are currently not considered standard of care and most can only be accessed via clinical trials. A list of clinical trials in advanced prostate cancer can be found here.
You should ask your clinician if you are suitable to take part in such a study or contact the relevant trial team directly.
- ii) Treating the prostate cancer deposits
There is some new evidence that cancer deposits can communicate with each other independently. Therefore, some clinicians are exploring whether there is any benefit in treating specific areas of cancer outside of the prostate in addition to medical therapy. This can be completed in the form of surgery and highly-precise radiotherapy (SABR or brand names such as Cyberknife®).
At present this form of treatment is only available in clinical trials in the UK (e.g. IP2-ATLANTA, CORE). However, similar to treating the prostate, men with a few or limited (oligometastatic) number of cancer deposits may get benefit from treating these areas.
Follow the above link again for a list of clinical trials in advanced prostate cancer. Once again, you should ask your clinician if you are suitable to take part in such a study or contact the relevant trial team directly
Other forms of metastatic prostate cancer
We have not covered the following forms of advanced (metastatic) prostate cancer. Firstly, men who have been diagnosed with metastatic prostate cancer that appears following previous treatment to their prostate that was intended to be curative (i.e. previous prostatectomy or radiotherapy). This is known as relapsing disease (sometimes referred to as “metachronous” or “oligorecurrent” disease). Secondly, men who have had advanced (metastatic) prostate cancer that is no longer responding to hormone therapy. This is known as “castrate-resistant” metastatic prostate cancer.
Both these have separate pathways for treatments and involve an individual discussion with your clinician. However, you may be offered some treatments we have discussed here.
Relevant Scientific References
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi: 10.1016/S0140-6736(15)01037-5 [doi].
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121-131.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352-360.
- Sweeney CJ, Chen Y, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737-746.
- England N. Clinical commissioning policy statement: Docetaxel in combination with androgen deprivation therapy for the treatment of hormone naıve metastatic prostate cancerНNHS england reference:[B15/PS/a]: NHS england. Redditch, UK: NHS England. 2016.
- Connor MJ, Shah TT, Horan G, Bevan CL, Winkler M, Ahmed HU. Cytoreductive treatment strategies for de novo metastatic prostate cancer. Nature Reviews Clinical Oncology. 2019:1-15.
- Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: Results of a feasibility and case-control study. J Urol. 2015;193(3):832-838.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366. doi: S0140-6736(18)32486-3 [pii].
- Connor MJ, Smith A, Miah S, et al. Targeting oligometastasis with stereotactic ablative radiation therapy or surgery in metastatic hormone-sensitive prostate cancer: A systematic review of prospective clinical trials. European Urology Oncology. 2020.

Prostate matters is a not for profit organisation that is committed to providing free expert advice about prostate issues from leading Clinical Authorities
PROSTATE MATTERS
FOLLOW US
Copyright Disclaimer: We try to acknowledge copyright as appropriate. If we have used something without acknowledging copyright, this is inadvertent. Please let us know by emailing info@prostatematters.co.uk


